Ethylene Glycol Antifreeze
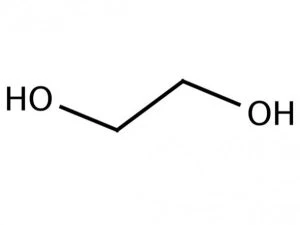
Ethylene Glycol Antifreeze / Monoethylene Glycol (C2H6O2) is a clear, colourless, odourless, slightly viscous liquid. It is miscible in both water and alcohol. It is the most important of the commercially available ethylene glycols as it has many industrial applications. Monoethylene Glycol is produced by the oxidation of ethylene at a high temperature in the presence of a silver oxide catalyst. The ethylene oxide is then hydrated to yield mono ethylene glycol with di and tri ethylene glycols as co-products.
C2H4O + H2O --> C2H6O2
Primary uses of ethylene glycol
Chemtex Ethylene Glycol Antifreeze is a vital ingredient in the production of polyester fibres, films, and resins, one of which is polyethylene terephthalate (PET). PET is used for making of plastic bottles. Ethylene Glycol has renowned antifreeze applications, being a major component in the manufacture of antifreeze, coolants, and de-icing liquids. Humectant properties make it ideal for use in the fibre treatment of textiles, pulp and paper industry, in adhesives, inks, and cellophane. It is also a used as a dehydration agent in natural gas pipelines, inhibiting the formation of natural gas clathrates before being recovered from the gas and reused.
Ethylene glycol is a useful industrial compound found in many consumer products. Examples include antifreeze, hydraulic brake fluids, some stamp pad inks, ballpoint pens, solvents, paints, plastics, films, and cosmetics. It can also be a pharmaceutical vehicle. Ethylene glycol has a sweet taste and is often ingested by accident or on purpose. Ethylene glycol breaks down into toxic compounds in the body. Ethylene glycol and its toxic byproducts first affect the central nervous system (CNS), then the heart, and finally the kidneys. Ingesting enough can cause death. Ethylene glycol is odorless.
Common Names of Ethylene Glycol:
- 1,2-Dihydroxyethane
- 1,2-Ethanediol
- Glycol
Physicochemical Data
| Properties | Typical Value |
| Form | Clear liquid |
| Appearance | Colourless |
| Odour | Odourless |
| CAS No. | 107-21-1 |
| Monoethylene Glycol Content | 99.5% min. |
| Solubility | Completely soluble with water |
Application Areas
Chemical Intermediates
- Solvents
- Polyester resins
- Resin esters as plasticizers
- Alkyl d-type resins
Humectant
- Textile fibers
- Paper
- Leather
- Adhesives
- Glue
Solvent Coupler
- Stabilizer against gel formation
- Freezing Point Depression
- Deicing fluids
- Heat transfer fluids (gas compressors, heating, ventilating, air conditioning, process chillers, ice rinks)
- All-weather automotive antifreeze and coolants
- Water-based formulations (adhesives, latex paints, asphalt emulsions)
Get Quote
Get a free quote for Ethylene Glycol Antifreeze liquid
