Sulfamic Acid Descalant
Chemtex Speciality Limited manufactures sulfamic acid in Industrial Grade, GP Grade and also with inhibitors, which finds wide acceptance across as an acid cleaning agent, for descaling metals and ceramics, cleaning boilers, heat exchangers, condenser jackets and coils, also for descaling toilets, removing excess grout on tiles, efflorescence and other mineral deposits.
To be used either in neat or in dilution, for removal of rust and limescales, replacing the use of more volatile and irritating hydrochloric acid. Often a component of household descaling agents or detergents for the removal of limescale, Sulfamic Acid has desirable water descaling properties, low volatility, and low toxicity. It forms water-soluble salts of calcium and ferric iron.
Sulfamic or sulphamic acid is preferable to hydrochloric acid in household use, due to its intrinsic safety. If erroneously mixed with hypochlorite based products such as bleach, it does not form chlorine gas, whereas the most common acids would; the reaction (neutralization) with ammonia, produces a salt.
Key Features and Benefits
- Effective removal of hard scale deposits
- Economical & cost effective dosage
- Safe for system metal and components
- Excellent wetting characteristics to aid in removal of organic and greasy foulants
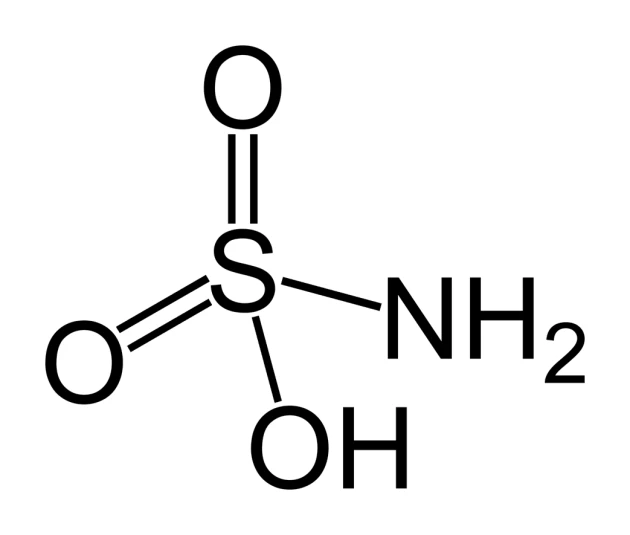
Sulfamic Acid | CAS No. 5329-14-6
| Properties | Typical Value |
| Form and Appearance | White Powder |
| Assay | 99.5% wt. (Min.) |
| Sulphate Content | 0.1% wt. (Max.) |
| Iron Content | 0.01% wt. (Max.) |
| Moisture Content | 0.01% wt. (Max.) |
| Insoluble Matter Content | 0.05% wt. (Max.) |
| Solubility | Soluble in cold water; Insoluble in organic solvents |
Sulfamic Acid GP Grade | CAS No. 5329-14-6
| Properties | Typical Value |
| Form and Appearance | White Powder |
| Assay | 98.0% wt. (Min.) |
| Sulphate Content | 1.0% wt. (Max.) |
| Iron Content | 0.1% wt. (Max.) |
| Moisture Content | 1.0% wt. (Max.) |
| Insoluble Matter Content | 0.3% wt. (Max.) |
| Solubility | Soluble in cold water; Insoluble in organic solvents |
Chemtex Chemclean Series
Inhibited Sulfamic Acid based Descalant. Aided with corrosion inhibitors, wetting agents and other chemicals make it a versatile descaling agent safe for system metal and components and safer in terms of equipment corrosion and safety of personnel.
